ABSTRACT
The Mānuka tree is unique to Aotearoa New Zealand and was recognised by early Māori traditions as a taonga (treasure) due to its wide variety of uses and is the first globally recognised taonga. Mānuka honey (MH) is reported to have exceptional antioxidant and antimicrobial properties. The objective of this review is to summarise the current published peer-reviewed literature for benefits of the use of MH as a Rongoā (traditional medicine) in ruminant livestock and companion animals to support the potential use of MH as a therapeutic for companion animals and livestock. The key finding was that there is very little peer-reviewed literature providing scientific evidence for the use of MH as a therapeutic in livestock and companion animals. There are a wide range of products now commercially available that have MH as an active ingredient. However, further scientific studies are required to evaluate the direct effects of MH relative to its effects when used in conjunction with other compounds, dose–response effects, and the effect of the Unique Mānuka Factor and methylglyoxal levels on efficacy in order to provide scientific evidence to support the efficacy of MH as a functional food or therapeutic in livestock and companion animal species.
For the full article, citation list, tables and author details, please refer to the online publication: https://www.tandfonline.com/doi/full/10.1080/00288233.2023.2274393?src=exp-la
Introduction
Sustainable resource use, ecological production, equitable distribution of provisions and community wellbeing are cornerstones of the traditional Māori way of life, founded in Kawa (etiquette) and Tikanga (culture). Māori landowners want to optimise their land use and connect their supply to customers via a ‘product’ that is grounded in Te Ao Māori. A key goal is to maximise a wide range of ‘values’ (environmental, cultural, social, and economic), and express kaitiakitanga (stewardship) over their natural resources, identity, and future.
Mānuka (Leptospermum scoparium) is unique to Aotearoa New Zealand and was recognised by early Māori traditions as a taonga (treasure) due to its wide variety of uses (Bil Citation2016). Mānuka is the first taonga that has gone global. Māori have a cultural connection to Mānuka based on our whakapapa. Tane Mahuta separated his parents Ranginui and Papatuānuku and through various unions gave rise to Mānuka and other native species.
Aotearoa New Zealand agriculture is under increasing pressure to reduce chemical and drug input and the farming community is seeking solutions to enhance animal health, wellbeing, and productivity through natural products. Furthermore, incentives are needed to encourage Māori landowners to develop underutilised whenua and new business opportunities are needed to support sustainable land use, economic growth, and community wellbeing.
The production of honey and its related products for domestic and export markets is an important economic activity in Aotearoa New Zealand. Zuchetta et al. (Citation2022) recently reviewed the scientific literature, technical literature and traditional knowledge databases describing the chemical properties and biological activity of four New Zealand monofloral honeys to support the visions and aspirations of Māori. Mānuka honey (MH) is the most prominent with well-established uses in humane medicine, however, its use in veterinary medicine is relatively new. Thus, there is limited science-based evidence for benefits of the use of MH in livestock or companion animal species. The aim of this review is to summarise the current peer-reviewed literature on the potential value proposition for use of MH as Rongoā for animals, notably livestock species (e.g. cattle, sheep, alpaca, pigs, horses) and companion animals (dogs and cats).
Rongoā Māori
Rongoā Māori is the traditional healing system of the indigenous Māori people of New Zealand (Ahuriri-Driscoll Citation2014; Mark et al. Citation2017). Rongoā is rooted in ancient knowledge passed down through generations and embodies the use of plants and other natural resources as well as spiritual healing, massage and incantations (the power of karakia) to promote physical, spiritual and emotional healing. Tohunga Rongoā (practitioners) consider both the underlying causes of an ailment (e.g. environment, lifestyle, spiritual state) and the symptoms to address imbalances and restore equilibrium. Rongoā also fosters a profound connection with nature with Tohunga Rongoā drawing on the wisdom of the land and indigenous plants which are taonga (treasures) with inherent healing properties. The sustainable and respectful use of these resources reflects the Māori belief in reciprocity with the natural world. This ancient practice continues to enrich the lives of Māori communities, preserving their traditions and promoting well-being in a comprehensive and meaningful way.
Rongoā Rākau (tree or wood) is a specific branch of Rongoā that focuses on the medicinal use of native plants and trees (Williams Citation2001). Identifying and using various parts of trees including leaves, bark, roots and resins to create remedies for a wide range of health conditions is a key part of Rongoā Rākau. The Tohunga Rongoā possesses an intricate understanding of the healing properties of different native plant species and when and how to harvest and process them. They have passed down this knowledge through generations, ensuring the preservation of their traditional healing practices. Many indigenous plant species possess unique medicinal attributes, and the preparation of remedies involves specific rituals and protocols to harness their healing powers effectively.
Classification and biological properties of MH
Mānuka honey is a unique mono-floral honey obtained from the Mānuka myrtle tree that belongs to the Myrtaceae family which grows as a shrub or small tree throughout New Zealand and Eastern Australia (Brophy et al. Citation2000). MH can be classified according to two properties: Unique Mānuka Factor (UMF) and methylglyoxal levels (MGO). The UMF factor is based on its antiseptic properties and can range from 5 + to 28 + mg/kg. MGO is the primary non-peroxide antibacterial constituent with quantities ranging from 83 + to 1450 + mg/kg. A range of carbohydrates and polyphenolic compounds are also implicated in the classification of MH (Alvarez-Suarez et al. Citation2014). Mānuka honey has a high osmolarity, low pH (3.5-4.5), and high sugar content which inhibits microbial growth (Brosseau et al. Citation2020).
Mānuka honey is highly valued for its non-peroxide anti-bacterial properties, largely attributed to its exclusive MGO content, is rich in a wide range of macro- and micro-nutrients and has been found to confer various health benefits credited to its unique chemical composition (reviewed by El-Senduny et al. Citation2021). Honey, including MH, is a natural product enriched with multiple phenolic compounds, enzymes and sugars with antioxidant, anticarcinogenic, anti-inflammatory, and antimicrobial properties (Carter et al. Citation2016; Scepankova et al. Citation2021). One of the primary uses of MH in veterinary medicine is in wound healing because of these antimicrobial properties.
Wound healing
Wound healing is a complex multi-stage process whereby the integrity and function of the tissue are restored (Fossum Citation2012; Scepankova et al. Citation2021). A wide range of topical treatments are commercially available to support wound healing, with honey as the active ingredient. Based on small animal studies, honey confers specific benefits for wound healing including acceleration of dermal repair and epithelialization, angiogenesis promotion, immune response promotion and a reduction in healing-related infections with pathogenic microorganisms (Scepankova et al. Citation2021). Mānuka honey has been reported to have therapeutic advantages in wound healing compared to other honeys due to its potent antibacterial and antioxidant properties (Alvarez-Suarez et al. Citation2014; Taskandi Citation2021). The reported beneficial effects of MH on wound healing in humans are postulated to be due to the stimulation of macrophage migration, increasing tissue turnover and the creation of a protective surface barrier that promotes healing (Alvarez-Suarez et al. Citation2014). MH may also have pro-inflammatory effects increasing the release of cytokines from leucocytes that regulate angiogenesis and fibroblast proliferation along endothelial cells based on horse (Bischofberger et al. Citation2013) and rat studies (Nooh and Nour-Eldien Citation2016). Lu et al. (Citation2019) also reported that when used at appropriate concentrations, Mānuka honeybased wound dressings may provide a promising treatment for infected chronic wounds including those with P. aeruginosa biofilms. Honey-based treatment strategies for burns and infected wounds based on recent pre-clinical research have recently been reviewed by Krishnakumar et al. (Citation2020).
Medical grade honey (MGH) is a term used by healthcare professionals to refer to honey used in wound treatment (Maruhashi Citation2020) and must fulfil specific criteria (reviewed by Taskandi Citation2021). Medical grade honey is reported to be a promising wound therapy due to its wide spectrum of antimicrobial efficacy with no known resistant strains and is effective against clinical bacterial and fungal isolates and their associated biofilm formulation (Taskandi Citation2021). It is also cost-effective, safe and is considered to be a potential alternative to antibiotics or complementary therapy for treatment of locally infected wounds (Taskandi Citation2021). Most commercially available MGH are formulated with MH as it is one of the most well-studied honey varieties in the world and is the first honey type to obtain the status of MGH (Scepankova et al. Citation2021).
Ruminant/pseudo-ruminant species
The peer-reviewed literature on the potential therapeutic evidence for MH in ruminant/pseudo-ruminant species was sourced using the publicly available bibliographic databases Scopus, PubMed and Google Scholar. All articles where MH was evaluated in ruminants were included in. In the review by Vogt et al. (Citation2021), there was only one study in ruminant species where potential effects of MH on wound healing were reported (Paramasivan et al. Citation2014). Since that review, a further six studies in ruminants (goats, sheep and cattle) and one study in alpaca where MH has been used in wound healing treatments have been reported (). Chigerwe et al. (Citation2020) reported on the use of MH as part of a treatment regime to heal burns in sheep, goats and pigs following wildfire injuries, and Williams et al. (Citation2022) reported on chronic wound healing involving the use of MH in a goat. However, while MH was used as part of the treatment regime in both case studies, the specific effect of MH on wound healing was not directly measured. Iacopetti et al. (Citation2020) reported that MH treatment of open wounds in sheep through daily application of MH enhanced the healing process through promotion of cell proliferation and neovascularization, resulting in a pro-inflammatory effect. Immunomodulatory effects were also reported during the healing process. However, wound closure time was not influenced by MH application in that study. These authors recommended administration of MH to wounds that appear to be non-healing or chronic as MH has a long-term effect on the healing process.Poza et al. (Citation2023) reported on a case study evaluating response to antimicrobial therapy whereby MH was placed in the cavity following the removal of a masseter abscess in an adult alpaca. This animal successfully recovered, however as there was only one animal in the case study, and there was no comparison without MH, it is not possible to determine if MH contributed to wound healing in this case. Collectively, these studies highlight the potential benefits for MH in wound healing in livestock species, particularly for treatment of chronic or non-healing wounds. However, except for Iacopetti et al. (Citation2020), studies to date have been case studies and therefore do not provide a robust comparison of the potential wound healing benefits of MH relative to other topical treatments.
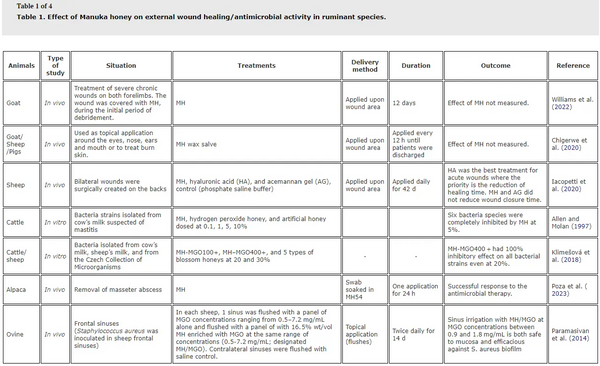
In vitro studies () have indicated anti-bacterial effects of MH (MGO400 + but not MH-MGO100 + or blossom honey) when applied to bacteria isolated from cow and sheep milk (Klimešová et al. Citation2018). Indeed, complete inhibition was observed in bacterial strains isolated in milk from cows suspected of having mastitis when 5% MH was applied (Allen and Molan Citation1997). Paramasivan et al. (Citation2014) reported that MH-MGO at concentrations between 0.9 and 1.8 mg/ml is safe and efficacious against S. aureus biofilm when used as a sinus irrigation. These studies highlight potential anti-bacterial effects of MH in ruminants. However, further in vivo studies are required to further explore the wound-healing and anti-microbial effects of MH in ruminants.
Monogastric livestock
The peer-reviewed literature on the potential therapeutic evidence for MH in monogastric species was sourced using the publicly available bibliographic databases Scopus, PubMed and Google Scholar. All articles reporting on the use of MH in monogastric livestock were included. The literature describing the potential effects of MH in wound healing in monogastric livestock species is presented in . The review by Vogt et al. (Citation2021) reported a range of responses to MH in the healing of created skin wounds in horses, including a more organised granulation tissue bed early in wound repair (Bischofberger et al. Citation2016), reduced wound retraction, and overall healing time (Bischofberger et al. Citation2013; Tsang et al. Citation2017). However, Bischofberger et al. (Citation2015) reported wound healing variables did not differ between MH gel, activated protein C, commercial antibiotic ointment, or no treatment control. Mandel et al. (Citation2020) reported that intralesional application of MGH (not specifically MH) can improve the repair of lacerations in horses. Healing times of contaminated equine distal limb wounds were improved following application of a MH gel (Bonifer-Engelhardt Citation2020), and surgical site infections have also been reported to be reduced following a single application of MH compared to untreated control wounds (McGovern and Bladon Citation2021). Horses that underwent colic surgery have also been reported to have reduced incisional infections following MH application to the linea alba prior to skin closure (Gustafsson et al. Citation2021). Anis et al. (Citation2021) also report improved healing of created skin wounds in donkeys when MH was included in a composite with many other ingredients, however specific effects of MH were not reported in that study. In contrast, McIver et al. (Citation2020) reported no effect of MH-UMF20, MH-UMF5, or MH-UMF5 + 1% cannabinol application to skin wounds contaminated with faeces in horses.
In pigs, Muhrbeck et al. (Citation2022) did not observe any difference in S. aureus count between topically applied MH 15 + or intramuscular gentamicin in wounds infected with S. aureus. Additionally, wound size was not reduced in the MH relative to gentamicin-treated animals. These observations suggest that MH dressings may be an alternative to antibiotic treatment to prevent the progression of wound infection, but not for accelerating healing. Similarly, Evensky et al. (Citation2019) reported no differences in wound healing when MH was incorporated into a resorbable membrane compared to control treatment for tissue regeneration in pigs.
Companion animals (dogs and cats)
The peer-reviewed literature on the potential therapeutic evidence for MH in companion animals (dogs and cats) was sourced using the publicly available bibliographic databases Scopus, PubMed and Google Scholar. All articles reporting on the use of MH in cats and dogs were included. In the review by Vogt et al. (Citation2021), only one study reported on the effect of MH on wound healing in companion animals. In that study, Apaydin et al. (Citation2019) reported that both MH and a 0.01% Ethacridine lactate (an antibacterial drug) antiseptic solution had similar effects on healing of infected wounds in cats. The authors recommended further clinical and experimental studies to be undertaken using microbiological, biochemical or histological parameters to further explore this finding. Since the review by Vogt et al. (Citation2021), a further 10 studies have been reported in cats and dogs ().
Dental diseases, and in particular periodontal disease, is the primary health condition affecting domestic cats (O’Neill et al. Citation2023) and dogs (O’Neill et al. Citation2021) often requiring tooth extraction. Pleeging et al. (Citation2022) reported that after extraction, intra-socket application of MGH resulted in decreased inflammation, improved mucoperiosteal flap viability and promoted wound healing at both 3- and 7-days post-extraction providing a potent adjuvant therapy to support intra-oral wound health.
Due to its antimicrobial activity and chemical properties that enhance wound healing and tissue regeneration (White Citation2016; Scepankova et al. Citation2021), MH is commonly used to support wound healing in dogs (e.g. Frey and Varjonen Citation2023). Repellin et al. (Citation2021) reported that a proprietary MH essential oil hydrogel application to small acute, full-thickness open wounds in healthy dogs did not differ in wound contraction and histological scores compared to control standard-of-care dressings. However, it was noted by the authors that there may be beneficial effects of this hydrogel in the early proliferative stage of wound healing and where wounds would benefit from early robust epithelialization. MH has also been found to be an alternative to the antibacterial drug Rivanol (Ethacridine lactate) in the treatment of infected wounds in cats (Apaydin et al. Citation2019).
Bacterial resistance to antibiotics is a growing global crisis that threatens the ability to treat infectious diseases in both humans and animals (Casadevall Citation2017). Intertrigo is a skin fold dermatitis that commonly requires recurrent treatment with topical antiseptics or antibiotics. Brosseau et al. (Citation2020) reported that MH was able to alleviate pruritus providing a mild short-term benefit, but was not able to treat the microbial, cytological and clinical components of the disease. As a result, the authors did not recommend the use of MGH as an alternative to topical antimicrobials in the control of canine nasal intertrigo. In contrast, Fregeneda-Grandes et al. (Citation2020) reported preliminary findings that suggest MGH may be effective in the control of clinical signs and microbial colonisation in dogs with otitis externa. Staphylococcus pseudintermedius (SP) is a gram-positive bacterium that can act as a skin commensal or a pathogen when the skin barrier is compromised. It is one of the main causative agents of canine pyoderma (Larsen et al. Citation2018) and can cause ear, wound and post-surgical infections in domestic animals, especially dogs (Bannoehr and Guardabassi Citation2012). In addition to its zoonotic potential, with the emergence of multidrug resistance, treating both animal and human SP infections can be difficult. Meroni et al. (Citation2020) reported that a MH gel and propolis had the lower minimum inhibitory concentration against 23 strains of SP isolated from canine pyoderma as compared to tea tree (Melaleuca alternifolia) and rosemary (Rosmarinus officinalis), highlighting the significant antibacterial effect of MH. In vitro, it has also been observed that MH at either 40 and 80% w/v concentrations was effective against common cutaneous pathogens Staphylococcus spp. (human and canine skin infections) and Pseudomonas spp. (canine otitis) (Cremers et al. Citation2020). Brown et al. (Citation2020) also reported that in vitro, MGH inhibited all 18 of the genetically diverse SP strains sequenced and tested in the study. Together, these studies highlight the potential for MH to be used against SP infections. Furthermore, when the effect of different honeys against certain bacterial/fungal ATCC strains and multidrug-resistant strains isolated from chronic otitis in dogs was evaluated, MH showed the greatest antimicrobial activity (Hulea et al. Citation2022).
Collectively, these studies provide supporting evidence for the potential veterinary application of MH, in particular MGH, for use in wound healing and as an alternative to traditional topical antibacterial and antibiotic formulations for treating skin conditions such as SP infections, and to prevent the progression of wound infection. There are a wide range of wound types (ulcers, burns, tooth extractions, injuries, surgical incisions, chronic wounds, etc.) that may require different interventions. Further studies are recommended to generate additional evidence for the veterinary use of MH in both livestock and companion animal species.
Gastrointestinal function
The review by Vogt et al. (Citation2021) described the supporting literature for potential anti-inflammatory, anti-tumorigenic, and anti-ulcer benefits of MH in murine species and various cell lines potentially through its anti-inflammatory and antioxidant properties. These studies highlight the potential for MH to have potentially beneficial effects on gastrointestinal function when ingested either as part of the diet (functional food) or when applied topically as a therapeutic. However, to the authors’ knowledge, there is no peer-reviewed literature describing the effect of MH on gastrointestinal function in livestock species (e.g. cattle, sheep, goats, horses) or companion animals.
Gastrointestinal disorders and associated morbidity and mortality are common in livestock and companion animals. Young stock are particularly affected, with a wide range of gastrointestinal disorders observed including abomasal damage in veal calves (Bus et al. Citation2019) and lambs (Vatn and Ulvund Citation2000), neonatal scours or enteritis in calves (Maier et al. Citation2022) and lambs (McCoard et al. Citation2021). However, adult animals are also affected, e.g. gastric ulceration in horses (van den Boom Citation2022), cattle (Munch et al. Citation2019) and sheep (Smith et al. Citation2023) and dogs (Davis and Williamson Citation2016). There is a wide range of causative factors including diet quality, diet type (especially rapidly fermentable diets), feeding management, stressful conditions, nonsteroidal anti-inflammatory drug administration, and infectious disease. Treatment options currently include electrolyte therapy (e.g. for non-infectious scours) or administration of drug therapies. Therefore, alternative preventative and/or therapeutic approaches are of significant interest.
Evidence from rodent models suggests that MH may have a beneficial effect on gastrointestinal function. Prakash et al. (Citation2008) reported that oral treatment with 5 g/kg or 10 g/kg body weight of MH was able to reduce colonic inflammation in an inflammatory model of colitis. In that study, all biochemical parameters were reduced, and lipid peroxidation was restored and antioxidant parameters improved. Almasaudi et al. (Citation2016) demonstrated that MH (2.5 mg/kg) provides gastroprotective effects in an acute (ethanol-induced) gastric ulcer model in rats by protecting the enzymatic and nonenzymatic antioxidants, maintaining glycoprotein, inhibiting lipid peroxidation, and reducing inflammatory cytokine production. These authors also reported that oral treatment with MH (2.5 mg/kg) in rats with acetic acid-induced gastric ulcers has potent antiulcer activity that may be due to its antioxidant abilities that interfere with the inflammatory process and reduces lipid peroxidation Almasaudi et al. (Citation2016). Whether similar beneficial effects observed in murine species can be demonstrated in livestock and companion animal species remains to be investigated.
Other physiological responses
There is very little peer-reviewed literature that suggests that MH may have other physiological benefits to improve health, wellbeing and/or productivity in livestock or companion animals (). Whyte et al. (Citation2017) reported that a commercially available combination of MH and aloe vera (Cow and Calf Formula, DairyCare Ltd, NZ) fed to dairy cattle improved milk production by 5.5% during peak lactation and 4.7% throughout the whole season. These authors postulated that this combination may have improved nutrient availability for milk production, however, no supporting data to demonstrate the mechanisms of action were reported. Positive effects on teat condition have also been reported with daily treatment of teats in lactating dairy cows with 0.5% iodine/14.5% emollient MH vs. 0.5% iodine/5% emollient (Thomas and Nydam Citation2011).
It is important to highlight that with the exception of one study in horses (McIver et al. Citation2020), neither UMF or MGO levels are reported in any of the animal studies that evaluated MH effects on sheep (Chigerwe et al. Citation2020; Iacopetti et al. Citation2020), goats (Williams et al. Citation2022), alpaca (Poza et al. Citation2023), pigs (Evensky et al. Citation2019; Chigerwe et al. Citation2020; Muhrbeck et al. Citation2022), dairy cattle (Allen and Molan Citation1997; Thomas and Nydam Citation2011; Whyte et al. Citation2017), cats (Apaydin et al. Citation2019; Pleeging et al. Citation2022), dogs (Brosseau et al. Citation2020; Brown et al. Citation2020; Cremers et al. Citation2020; Fregeneda-Grandes et al. Citation2020; Meroni et al. Citation2020; Repellin et al. Citation2021; Hulea et al. Citation2022; Frey and Varjonen Citation2023) and horses (Bonifer-Engelhardt Citation2020; Mandel et al. Citation2020; De Clercq et al. Citation2021; Gustafsson et al. Citation2021; McGovern and Bladon Citation2021;). While the biological activity of MH increases as UMF increases, there is no clear evidence that the effect of the MH on wound healing and other physiological responses in livestock species or companion animals is related to the UMF or MGO. There are reports suggesting that the antibacterial properties are associated with the MGO or UMF (Georgescu et al. Citation2017), however, others reported that such association does not exist (Girma et al. Citation2019; Muhrbeck et al. Citation2022). Curtis (Citation2018) suggests that the MH used for wound management needs to have a minimum of 10+ UMF. Muhrbeck et al. (Citation2022) suggested that the antimicrobial effect of MH was through hydrogen peroxide or other phytochemical components rather than through MGO. Girma et al. (Citation2019) suggested that, as the MGO is generated from dihydroxyacetone (DHA) during the maturation of honey for at least one year, the estimated UMF may not reflect the actual MGO content at the time of use of the MH. Therefore, MH’s MGO or UMF content will vary with the time of its estimation and that may be the cause of lack of effect observed when comparing MHs with different UMF levels. Further studies are required to determine whether the UMF or MGO levels are related to the level of activity and/or efficacy of MH and whether minimum thresholds exist.
Conclusion
Based on the findings of this literature review, there is good supporting peer-reviewed literature for the antibacterial action of MH to prevent and/or manage infection, and some supporting evidence for wound healing in livestock and companion animal species. However, the biological mechanisms of action have not been elucidated. Furthermore, whether the UMF or MGO indexes influence the effects of MH has not been scientifically evaluated. Based on murine and in vitro studies, there is significant potential for MH to have beneficial effects beyond wound healing, especially for improving gastrointestinal function and alleviating inflammation, and well as potential productivity effects (e.g. increased milk yield). There are a wide range of products now commercially available that have MH as an active ingredient. However, further scientific studies are required to evaluate the direct effects of MH relative to its effects when used in conjunction with other compounds, dose–response effects, and the effect of UMF and MGO levels on efficacy to provide scientific evidence to support the efficacy of MH as a functional food or therapeutic in livestock and companion animal species. If these benefits can be proven, MH may provide an important alternative to drug therapies to support health, wellbeing, and performance. However, potential risk factors and/or negative effects have been poorly explored and warrant further investigation. Collectively, the evidence generated from scientific studies to date highlights future opportunities to promote the use of MH in Rongoā and support the growth and development of MH-based enterprises for Iwi, hapū and whanau. We advocate that randomised controlled trials are undertaken in both livestock and companion animal species to determine efficacy and safety of MH in these species.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahuriri-Driscoll A. 2014. He kōrero wairua: indigenous spiritual inquiry in rongoā research. MAI Journal. 3(1):33–43. [Google Scholar]
- Allen KL, Molan PC. 1997. The sensitivity of mastitis-causing bacteria to the antibacterial activity of honey. New Zealand Journal of Agricultural Research. 40(4):537–540. doi:10.1080/00288233.1997.9513276. [Taylor & Francis Online] [Web of Science ®], [Google Scholar]
- Almasaudi SB, El-Shitany NA, Abbas AT, Abdel-dayem UA, Ali SS, Jaouni SK, Harkeh S. 2016. Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. Oxidative Medicine and Cellular Longevity. 2016:3643824. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Alvarez-Suarez JM, Gasparrini M, Forbes-Hernandez TY, Mazzoni I, Giampieri F. 2014. The composition and biological activity of honey: a focus on Manuka honey. Foods (basel, Switzerland). 3(3):410–432. [Google Scholar]
- Anis A, Sharshar A, El Hanbally S, Sadek Y. 2021. A novel organic composite accelerates wound healing: experimental and clinical study in equine. Journal of Equine Veterinary Science. 99:103406. doi:10.1016/j.jevs.2021.103406. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Apaydin N, Kemiksiz E, Akcay A. 2019. Comparison of Manuka honey (Manuka Nd, G) and Etacridine Lactate (Rivanol) applications in the treatment of infected wounds in cats. Acta Scientiae Veterinariae. 47:1643. [Web of Science ®], [Google Scholar]
- Bannoehr J, Guardabassi L. 2012. Staphylococcus pseudintermedius in the dog: taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Veterinary Dermatology. 23:253–e52. doi:10.1111/j.1365-3164.2012.01046.x. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Bil G. 2016. Between Māori and modern? The case of Manuka honey. appreciating local knowledge. Newcastle, UK: Cambridge Scholars Publishing; p. 61. [Google Scholar]
- Bischofberger A, Dart CM, Horadagoda N, Perkins NR, Leffcott LB, Little CB, Dart AJ. 2016. Effect of Manuka honey gel on the transforming growth factor β1 and β3 concentrations, bacterial counts and histomorphology of contaminated full-thickness skin wounds in equine distal limbs. Australian Veterinary Journal. 94(1-2):27–34. doi:10.1111/avj.12405. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Bischofberger A, Tsang AS, Horadagoda N, Dart CM, Perkins NR, Jeffcott LB, Jackson CJ, Dart AJ. 2015. Effect of activated protein C in second intention healing of equine distal limb wounds: a preliminary study. Australian Veterinary Journal. 93(10):361–366. doi:10.1111/avj.12363. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Bischofberger AS, Dart CM, Perkins NR, Diplomate ACT, Dart AJ. 2011. A preliminary study on the effect of manuka honey on second intention healing of wounds on distal forelimbs in horses. Veterinary Surgery. 40:898–902. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Bischofberger AS, Dart CM, Perkins NR, Kelly A, Jeffcott L, Dart AJ. 2013. The effect of short- and long-term treatment with manuka honey on second intention healing of contaminated and noncontaminated wounds on the distal aspect of the forelimbs in horses. Veterinary Surgery. 42:154–160. doi:10.1111/j.1532-950X.2012.01083.x. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Bonifer-Engelhardt E. 2020. Manuka honey gel as a viable topical treatment to decrease contaminated equine distal limb wound healing times. Science Extension Project Report. [Google Scholar]
- Brophy JJ, Goldsack RJ, Bean AR, Forster PI, Lepschi BJ. 2000. Leaf essential oils of the genus Leptospermum (Myrtaceae) in eastern Australia. Part 6. Leptospermum polygalifolium and allies. Flavour and Fragrance Journal. 15:271–277. https://doi.org/10.1002/1099-1026(200007/08)15:4<271::AID-FFJ910>3.0.CO;2-E. [Crossref] [Web of Science ®], [Google Scholar]
- Brosseau G, Page N, de Jaham C, del Castillo JRE. 2020. Medical honey for canine nasal intertrigo: A randomized, blinded, placebo-controlled, adaptive clinical trial to support antimicrobial stewardship in veterinary dermatology. PLoS ONE. 15(8):e0235689. doi:10.1371/journal.pone.0235689. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Brown HL, Metters G, Hitchings MD, Wilkinson TS, Sousa L, Cooper J, Dance H, Atterbury RJ, Jenkins R. 2020. Antibactgerial and antivirulence activity of manuka honey against genetically diverse Staphylococcus pseudintermedius strains. Applied and Environmental Micrology. 86(20):e01768. [PubMed] [Web of Science ®], [Google Scholar]
- Bus JD, Stockhofe N, Webb LE. 2019. Invited review: abomasal damage in veal calves. Journal of Dairy Science. 102:943–960. doi:10.3168/jds.2018-15292. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Carter DA, Blair SE, Cokcetin NN, Bouzo D, Brooks P, Schothauer R, Harry EJ. 2016. Therapeutic Manuka honey: no longer so alternative. Frontiers in Microbiology. 7:569. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Casadevall A. 2017. Crisis in infectious diseases: 2 decades later. Clinical Infectious Diseases. 64(7):823–828. doi:10.1093/cid/cix067. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Chigerwe M, Depenbrock SM, Heller MC, King A, Clergue SA, Morris CM, Peyton JL, Angelos JA. 2020. Clinical management and outcomes for goats, sheep, and pigs hospitalized for treatment of burn injuries sustained in wildfires: 28 cases (2006, 2015, and 2018). Journal of the American Veterinary Medical Association. 257(11):1165–1170. doi:10.2460/javma.2020.257.11.1165. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Cremers N, Belas A, Costa SS, Couto I, de Rooster H, Pomba C. 2020. In vitro antimicrobial efficacy of two medical grade honey formulations against common high risk meticillin-resistant staphylococci and Pseumodomonas spp. pathogens. Veterinary Dermatology. 31:90. doi:10.1111/vde.12811. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Curtis A. 2018. Why use manuka honey? The Veterinary Nurse. 9(10). doi:10.12968/vetn.2018.9.10.513. [Crossref], [Google Scholar]
- Davis MS, Williamson KK. 2016. Gastritis and gastric ulcers in working dogs. Frontiers in Veterinary Science. 3:30. doi:10.3389/fvets.2016.00030. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- De Clercq E, Den Hondt S, De Baere C. 2021. Effects of various wound dressings on microbial growth in perfused equine musculocutaneous flaps. American Journal of Veterinary Research. 82(3):189–197. doi:10.2460/ajvr.82.3.189. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- El-Senduny FF, Hegazi NM, Elghani GE, Farag MA. 2021. Manuka honey, a unique mono-floral honey. A comprehensive review of its bioactives, metabolism, action mechanisms, and therapeutic merits. Food Bioscience. 42:101038. doi:10.1016/j.fbio.2021.101038. [Crossref] [Web of Science ®], [Google Scholar]
- Evensky JA, Green MS, Stein SH, Bowlin GL, Hollis W. 2019. Regenerative properties of a Manuka honey incorporated membrane in a porcine model. Online Journal of Dentistry & Oral Health. 1(4):1–7. doi:10.33552/OJDOH.2019.01.000520. [Crossref], [Google Scholar]
- Fossum T. 2012. Small animal surgery. 4th ed. Missouri (USA): Mosby Elsevier. [Google Scholar]
- Fregeneda-Grandes JM, Nicolas-Gonzalez JJ, Rejas-Lopez J, Carvajal-Uruena A. 2020. Preliminary evaluation of two commercial ear solutions in the treatment of canine otitis externa. Journal of Small Animal Practice. 61(9):547–553. doi:10.1111/jsap.13177. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Frey R, Varjonen K. 2023. A retrospective case series of the postoperative outcome for 30 dogs with inflammatory interdigital nodules, surgically treated with carbon dioxide laser and a nonantimicrobial wound-healing protocol. Veterinary Dermatology. 34:150–155. doi:10.1111/vde.13146. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Georgescu M, Dobrea M, Dobrea VC. 2017. Antimicrobial effect of commercial Manuka honey and conventional local honey against gram-negative and gram-positive bacteria. Scientific Works (Series C Veterinary Medicine). 63(2):133–136. [Google Scholar]
- Girma A, Seo W, She RC. 2019. Antibacterial activity of varying UMF-graded Manuka honeys. PLos ONE. 14(10):e0224495. doi:10.1371/journal.pone.0224495. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Gustafsson K, Tatz AJ, Slavin RA, Sutton GA, Dahan R, Ahmad WA, Kelmer G. 2021. Intraincisional medical grade honey decreases the prevalence of incisional infection in horses undergoing colic surgery: A prospective randomised controlled study. Equine Veterinary Journal. 53:1112–1118. doi:10.1111/evj.13407. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Herdan CL, Acke E, Dicken M, Archer RM, Forsyth SF, Gee EK, Pauwels FET. 2012. Multi-drug-resistant Enterococcus spp. As a cause of non-responsive septic synovitis in three horses. New Zealand Veterinary Journal. 60(5):297–304. doi:10.1080/00480169.2011.651702. [Taylor & Francis Online] [PubMed] [Web of Science ®], [Google Scholar]
- Hulea A, Obistioiu D, Cocan I, Alexa E, Negrea M, Neassu A-G, Hulea C, Pascu C, Costinar L, Iancu I, et al. 2022. Diversity of monofloral honey based on the antimicrobial and antioxidant potential. Antibiotics. 11:595. doi:10.3390/antibiotics11050595. [Crossref], [Google Scholar]
- Iacopetti I, Perazzi A, Martinello T, Gemignani F, Patruno M. 2020. Hyaluronic acid, Manuka honey and Acemannan gel: wound-specific applications for skin lesions. Research in Veterinary Science. 129:82–89. doi:10.1016/j.rvsc.2020.01.009. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Klimešová M, Němečková I, Vondrušková E, Nejeschlebová L. 2018. Antibacterial effect of Czech and Mānuka honey on selected mastitis pathogens. Acta Veterinaria Brno. 87:387–393. doi:10.2754/avb201887040387. [Crossref] [Web of Science ®], [Google Scholar]
- Krishnakumar GS, Mahendiran B, Gopalakrishnan S, Muthusamy S, Elangovan SM. 2020. Honey based treatment strategies for infected wounds and burns: A systematic review of recent pre-clinical research. Wound Medicine. 30:100188. doi:10.1016/j.wndm.2020.100188. [Crossref], [Google Scholar]
- Larsen RF, Boysen L, Jessen LR, Guardabassi L, Damborg P. 2018. Diversity of Staphylococcus pseudintermedius in carriage sites and skin lesions of dogs with superficial bacterial folliculitis: potential implications for diagnostic testing and therapy. Veterinary Dermatology. 29:291–e100. doi:10.1111/vde.12549. [Crossref] [Web of Science ®], [Google Scholar]
- Lu J, Cokcetin NN, Burke CM, Turnbull L, Liu M, Carter DA, Whitchurch CB, Harry EJ. 2019. Honey can inhibit and eliminate biofilms produced by Pseudomonas aeruginosa. Scientific Reports. 9:18160. doi:10.1038/s41598-019-54576-2. [Crossref] [PubMed], [Google Scholar]
- Maier GU, Breitenbuecher J, Gomez JP, Samah F, Fausak E, van Noord M. 2022. Vaccination for the prevention of neonatal calf diarrhea in cow-calf operations: a scoping review. Veterinary and Animal Science. 15:100238. doi:10.1016/j.vas.2022.100238. [Crossref] [PubMed], [Google Scholar]
- Mandel HH, Sutton GA, Abu E, Kelmer G. 2020. Intralesional application of medical grade honey improves healing of surgically treated lacerations in horses. Equine Veterinary Journal. 52(1):41–45. doi:10.1111/evj.13111. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Mark G, Chamberlain K, Boulton A. 2017. Acknowledging the Māori cultural values and beliefs embedded in rongoā Māori healing. International Journal of Indigenous Health. 12(1):75–92. doi:10.18357/ijih121201716902. [Crossref] [Web of Science ®], [Google Scholar]
- Maruhashi E. 2020. Honey in wound healing. In: Boateng J, editor. Therapeutic dressings and wound healing applications. US: Wiley; p. 235–254. [Crossref], [Google Scholar]
- McCoard SA, Hea S-Y, Karatiana D, Triggs J, Macdonald T. 2021. Comparison of milk replacer composition and effects on growth and health of preruminant lambs, and health-associated costs of artificial rearing. Applied Animal Science. 37:176–185. doi:10.15232/aas.2020-02093. [Crossref], [Google Scholar]
- McGovern KF, Bladon BM. 2021. Prophylactic treatment of equine laparotomy incisions with manuka honey may reduce the incidence of incisional infection in horses. Equine Veterinary Education. 33(Suppl 12):43. [Google Scholar]
- McIver VC, Tsang AS, Symonds NE, Perkins NR, Uquillas E, Dart CM, Jeffcott LB, Dart AJ. 2020. Effects of topical treatment of cannabidiol extract in a unique manuka factor 5 manuka honey carrier on second intention wound healing on equine distal limb wounds: a preliminary study. Australian Veterinary Journal. 98(6):250–255. doi:10.1111/avj.12932. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Meroni G, Cardin E, Rendina C, Millar VRH, Filipe JFS, Martino PA. 2020. In vitro efficacy of essential oils from Melaleuca Alternifolia and Rosmarinus Officinalis, Manuka honey-based gel, and propolis as antibacterial agents against canine Staphylococcus Pseudintermedius strains. Antibiotics. 9(6):344. doi:10.3390/antibiotics9060344. [Crossref], [Google Scholar]
- Muhrbeck M, Wladis A, Lampi M, Andersson P, Junker JPE. 2022. Efficacy of topical honey compared to systemic gentamicin for treatment of infected war wounds in a porcine model: A non-inferiority experimental pilot study. Injury. 53(2):381–392. doi:10.1016/j.injury.2021.10.019. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Munch SL, Nielsen SS, Krogh MA, Capion N. 2019. Prevalence of abomasal lesions in Danish Holstein cows at the time of slaughter. Journal of Dairy Science. 102:5403–5409. doi:10.3168/jds.2018-15757. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Nooh HZ, Nour-Eldien NM. 2016. The dual anti-inflammatory and antioxidant activities of natural honey promote cell proliferation and neural regeneration in a rat model of colitis. Acta Histochemica. 118(6):588–595. doi:10.1016/j.acthis.2016.06.006. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- O’Neill DG, Blenkarn A, Brodbelt DC, Church DB, Freeman A. 2023. Peridontal disease in cats under primary veterinary care in the UK: frequency and risk factors. Journal of Feline Medicine and Surgery. 25(3):1–12. doi:10.1177/1098612X231158154. [Crossref] [Web of Science ®], [Google Scholar]
- O’Neill DG, James H, Brodbelt DC, Church DB, Pegram C. 2021. Prevalence of commonly diagnosed disorders in UK dogs under primary veterinary care: results and applications. BMC Veterinary Research. 17:69. doi:10.1186/s12917-021-02775-3. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Paramasivan S, Brilling AJ, Jardeleza C, Jervis-Bardy J, Vreugde S, Wormald PJ. 2014. Methylglyoxal-augmented manuka honey as a topical anti-Staphylococcus aureus biofilm agent: safety and efficacy in an in vivo model. International Forum of Allergy & Rhinology. 4(3):187–195. doi:10.1002/alr.21264. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Pleeging CCF, de Rooster H, Van Wijk B, Wagener FADTG, Cremers NAF. 2022. Intra-socket application of medical-grade honey after tooth extraction attenuates inflammation and promotes healing in cats. Journal of Feline Medicine and Surgery. 24(12):e618–e627. doi:10.1177/1098612X221125772. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Poza MN, Nial C, Cajsa I, Alison T. 2023. Fusobacterium necrophorum and Actinomyces spp. masseter muscle abscessation in an adult alpaca. Veterinary Record Case Reports. 11(1):e521. doi:10.1002/vrc2.521. [Crossref], [Google Scholar]
- Prakash A, Medhi B, Avti PK, Saikia UN, Pandhi P, Khanduja KL. 2008. Effect of different doses of Manuka honey in experimentally induced inflammatory bowel disease in rats. Phytotherapy Research. 22(11):1511–1519. doi:10.1002/ptr.2523. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Repellin RL, Pitt KA, Lu M, Welder J, Norland EL, Stanley BJ. 2021. The effects of a proprietary manuka honey and essential oil hydrogel on the healing of acute full-thickness wounds in dogs. Veterinary Surgery. 50:1634–1643. doi:10.1111/vsu.13711. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Scepankova H, Combarros-Fuertes P, Fresno JM, Tornadijo ME, Dias MS, Pinto CA, Saraiva JA, Estevinho LM. 2021. Review: role of honey in advanced wound care. Molecules. 26:4784. doi:10.3390/molecules26164784. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Smith JS, Gebert J, Bennett K, Ebner LS, Flynn R, Mulon P-Y, Harvill L, Escher OG, Kreuder AJ, Bergman J, Cox S. 2023. The pharmacokinetics and pharmacodynamics of esomeprazole in sheep after intravenous dosing. Frontiers in Veterinary Science. 10:1172023. doi:10.3389/fvets.2023.1172023. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Taskandi H. 2021. Honey in wound healing: An updated review. Open Life Sciences. 16:1091–1100. doi:10.1515/biol-2021-0084. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Thomas MJ, Nydam DV. 2011. Evaluation of an Iodine-Based Manuka Honey Teat Dip on Teat End Health. in NMC 3rd International Symposium. 2011. St. Louis, Missouri, USA. [Google Scholar]
- Tsang AS, Dart AJ, Sole-Guitart A, Dart CM, Perkins NR, Jeffcott LB. 2017. Comparison of the effects of topical application of UMF20 and UMF5 manuka honey with a generic multifloral honey on wound healing variables in an uncontaminated surgical equine distal limb wound model. Australian Veterinary Journal. 95(9):333–337. doi:10.1111/avj.12616. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- van den Boom R. 2022. Invited review: equine gastric ulcer syndrome in adult horses. The Veterinary Journal. 105830:283–284. [Google Scholar]
- Vatn S, Ulvund MJ. 2000. Abomasal bloat, haemorrhage and ulcers in young Norwegian lambs. Veterinary Record. 146:35–39. doi:10.1136/vr.146.2.35. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Vogt NA, Nwosu A, Sargeant JM. 2021. A scoping review of the evidence for the medicinal use of natural honey in animals. Frontiers in Veterinary Science. 7:618301. doi:10.3389/fvets.2020.618301. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- White R. 2016. Manuka honey in wound management: greater than the sum of its parts? Journal of Wound Care. 25:539–543. doi:10.12968/jowc.2016.25.9.539. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]
- Whyte DK, Sharma K, Tarver P. 2017. The impact of feeding a supplement based on aloe and Manuka honey on milk yield from dairy cows. Journal of Applied Animal Nutrition. 5(e2):1–6. [Google Scholar]
- Williams DV. 2001. Maētauranga Maēori and taonga: the nature and extent of treaty rights held by iwi and hapuē in indigenous flora and fauna, cultural heritage objects, valued traditional knowledge. Wellington, New Zealand: Waitangi Tribunal. [Google Scholar]
- Williams NJ, Holter DL, Boileau MJ, Dugat DR. 2022. Use of meshed skin grafts to treat bilateral, circumferential skin defects of the antebrachium in a pygmy goat wether. Veterinary Record Case Reports. 10(2):e317. doi:10.1002/vrc2.317. [Crossref], [Google Scholar]
- Zuchetta C, Tangohau W, McCallion A, Hardy DJ, McCormick AC. 2022. Exploring the chemical properties and biological activity of four New Zealand monofloral honeys to support the Māori vision and aspirations. Molecules. 27:3282. doi:10.3390/molecules27103282. [Crossref] [PubMed] [Web of Science ®], [Google Scholar]